How to Convert Transmittance to Absorbance? | Different Methods for Converting
To convert percent transmittance to absorbance, there are two approaches. The first is the computation approach, while the second is the nomogram method. Transmittance values range from 0 to 1 and the percentage transmittance range from 0% to 100% where absorbance takes values from 0 to upwards.
The ratio of radiant power transmitted by a sample to radiant power incident on the sample is defined as transmittance. The negative logarithm of transmittance is absorption. Transmittance decreases exponentially. On the other hand, absorbance increases linearly.
Methods to Convert Transmittance to Absorbance
Absorbance and transmittance are measurements used in spectrophotometry. Spectrophotometry determines how much radiant energy a substance absorbs at different light wavelengths.
The technique is effective for determining the identity of an unknown material as well as determining the concentration of a substance in a sample using a set of standards. The ways to convert transmittance to absorbance are discussed below.
Method 1: Calculation Method
Absorbance and transmittance are related. Many substances absorb ultraviolet (UV) or visible light. The diagram below depicts a monochromatic radiation beam with radiant power P0 focused on a sample solution. Absorption occurs, and the radiation beam leaving the sample has radiant power P.
To convert percent transmittance to absorbance, use the relationships listed below.
A = -log T; T = 10^(-A)
The relationship between absorbance and transmittance
A = 2 – log %T; log %T = 2 – A; %T = 10^(2-A)
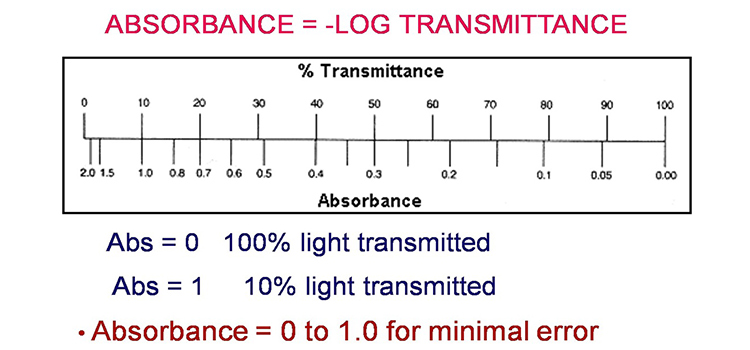
Method 2: Nomogram Method
The graph depicts a one-to-one relationship between absorbance and percent transmittance. If you know one of these two values, you may read the value corresponding to the other direction from the figure.
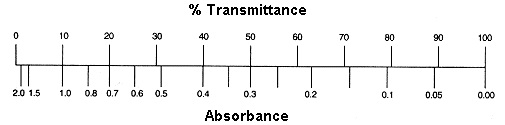
If all of the light passes through a solution without being absorbed, the absorbance is zero and the transmittance is 100 percent. If all of the light is absorbed, the transmittance percentage is zero, and the absorption is unlimited.
Method 3: Experimental Method
There is another method to transform admittance to absorbance. Take a sample of the appropriate percent T in a 1 cm cuvette, place it in a spectrophotometer, and read the absorbance of the sample straight from the instrument after subtracting the blank.
Convert Transmittance to Absorbance in Excel
A=-log absorption is the absorption measurement (percent T). You must undo the log function to convert the absorption values to transmission values. On Excel, an easy approach to achieve this is to add a new column so that you do not lose the original absorption data in your spreadsheet.
If your absorption data is in column B and starts on row 2, you should be able to convert it in cell C2 by entering the formula =(10(-B2))*100. This gives you the percentage T. You may then pull down on the lower right-hand corner of the cell, and the formula will automatically update the cell address of the data point.
Transmittance to Absorbance Table
In the lab or in the field, a transmittance to absorbance table allows for quick conversion of transmittance measurements to absorbance. Here, a table of transmittance to absorbance is given below.
| Transmittance | Absorbance (AU) |
| 100% | 0 |
| 99% | 0.004 |
| 98% | 0.009 |
| 97% | 0.013 |
| 96% | 0.018 |
| 95% | 0.022 |
| 90% | 0.046 |
| 85% | 0.071 |
| 80% | 0.097 |
| 75% | 0.125 |
| 70% | 0.155 |
| 65% | 0.187 |
| 60% | 0.222 |
| 55% | 0.26 |
| 50% | 0.301 |
| 45% | 0.347 |
| 40% | 0.398 |
| 35% | 0.456 |
| 30% | 0.523 |
| 25% | 0.602 |
| 20% | 0.699 |
| 15% | 0.824 |
| 10% | 1 |
| 5% | 1.301 |
| 1% | 2 |
Beer-Lambert Law
The Beer-Lambert law, often known as Beer’s Law, describes the relationship between absorbance (A), molar solute concentration in M (c), and the length of the light path to the sample in centimeters (l). Absorption is proportional to concentration and length: A = εcl.ε.
It is the wavelength-dependent molar absorptivity coefficient, which is constant for a given substance. ε has the units L mol – 1 cm – 1. Beer’s law describes a linear relationship between concentration and absorbance that may be plotted to create a simple graph.
What is the Relation Between Transmittance and Absorbance?
The inverse of absorbance is transmittance. Absorbance is the amount of light that a solution absorbs, whereas transmittance is the amount of light that goes through a solution.
The absorbance is proportional to the transmittance, with an absorbance of 0 equal to a transmittance of 100% and an absorbance of 1 equivalent to a transmittance of 10%. Because absorbance is a dimensionless quantity, it should be unitless.
Use of Transmittance
Transmittance measurement has a wide range of applications. Such as chemical concentrations in solutions are measured with transmittance. Also, it is used to test the clarity of the water. Syrup quality is also measured with it. Testing window tint films and glass clarity or haze in the atmosphere is also included.
Frequently Asked Questions
How do you calculate concentration from transmittance?
T = 1/10 transmission or transmittance
Transmission is calculated in a photometer by dividing the light that escapes by the light that enters the sample.
Absorption (A) = log (10/1).
The negative decadic logarithm of transmission is used to compute absorbance.
Concentration (C) = A/ (L x Ɛ) Absorbance (A) = C x L x Ɛ
The Lambert-Beer law defines the relationship of absorbance on sample concentration (C), optical path length (L), and a sample-specific extinction coefficient (Ɛ), which applies to a specific material at a specific wavelength. The concentration of the sample is then calculated by converting the formula.
Conclusion
For a completely transparent material, 100% of the light will travel through the other side of the barrier, and the transmittance is 100% for this type of material. On the other hand, a completely opaque material does not allow light to travel through it. As a result, the transmittance is 0%.
Subscribe to our newsletter
& plug into
the world of circuits