Is Hydrogen Conductive? – Let’s Learn about Hydrogen Conductivity
Hydrogen has the highest thermal conductivity of all gases. However, it is still regarded as a poor conductor of heat.
As for its electrical conductivity, it is a poor conductor of electricity under normal conditions. However, upon cooling hydrogen enough, it becomes a superconductor. Contrastingly, if heated enough, hydrogen becomes plasma and becomes highly conductive.
Does Hydrogen Have Conductivity or Not?
Despite being a nonmetal, hydrogen is placed above the first group in the periodic table. That is because it has an ns1 electron configuration like the alkali metals. Hydrogen remains a nonmetal even in that state.
The principle of conduction states that the electrons must move freely for conduction to take place. As hydrogen atoms hold on to their electrons tightly, it conducts heat and electricity poorly.
Heat Conduction
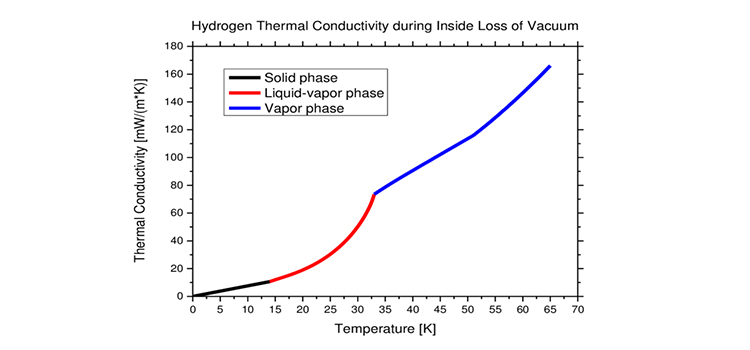
Thermal conductivity gives us an idea about a material’s capability to conduct heat. It is a material property and its higher values describe better heat conduction.
For most gases and vapors, the value of thermal conductivity is between 0.01 and 0.03 W/mK at room temperature. However, hydrogen is an exception. It has the highest thermal conductivity of any gas (0.18). The only one close is helium (0.15).
The reason behind such high thermal conductivity of hydrogen is because two isomers of hydrogen exist- ortho and para hydrogen. They stay in equilibrium with each other. The thermal conductivity of hydrogen depends on temperature and pressure.
At standard temperature and pressure, hydrogen gas has around 25% of the para form and 75% of the ortho form. At lower temperatures, the fraction of para-hydrogen is more dominant. Whereas at very low temperatures, the para form is more exclusively present. In the contrast, with the increase in temperature, the ortho fraction increases.
Electrical Conduction
Similar to thermal conductivity, the electrical conductivity of hydrogen depends on temperature. If hydrogen is cooled enough, it can be a superconductor. It can also be heated to a high temperature to make it exist as a plasma, which is highly conductive. If the pressure is high enough, hydrogen becomes a good conductor as well.
Is Hydrogen a Conductor or Insulator?
Hydrogen in its natural state is a perfect insulator. Similar gases such as oxygen, nitrogen, etc. are all poor conductors of heat. However, hydrogen becomes a very good conductor of electricity at very high pressure.
Experiments have shown that if the pressure is above 220 GPa, hydrogen becomes opaque and electrically conductive. At 260–270 GPa, hydrogen will transform into a metal. As a result, the conductance of hydrogen will increase sharply. Upon changing the pressure up to 300 GPa or cooling to at least 30 K, the sample will reflect light well.
Is Liquid Hydrogen a Conductor?
Under atmospheric conditions, hydrogen is a gas. However, if it is cooled below -423 degrees Fahrenheit, then it turns into a liquid. Another way of turning hydrogen into liquid is to squeeze it under immense pressure and high temperature.
In the liquid state, hydrogen molecules remain intact. Therefore, liquid hydrogen can be regarded as a poor conductor of electricity.
At ultracold temperatures, below -423 degrees Fahrenheit, hydrogen condenses into a liquid. It also turns into a liquid at higher temperatures when squeezed under immense pressure. As the molecules remain intact, this state of liquid hydrogen is a poor conductor of electricity.
Is Metallic Hydrogen Possible?
Metallic hydrogen is a particular phase of hydrogen in which it behaves like it conducts electricity. Research has shown that at high pressure and temperatures, metallic hydrogen can indeed exist as a partial liquid rather than a solid.
Moreover, the researchers think that it might be present in a huge amount of gravitationally compressed interiors of Jupiter, Saturn, and some exoplanets.
Is Metallic Hydrogen a Superconductor?
Up to room temperature, metallic hydrogen might stay as a superconductor. The theory was first suggested by Neil Ashcroft in 1968. There is an expected strong coupling between conduction electrons and lattice vibrations. The suggestion made was based on this.
In early 2019, this information has been confirmed at least twice as metallic hydrogen was made in the laboratory. Moreover, a 250K Meissner effect has been observed by Silvera et al. and a team in France.
The theory is that if very high pressures were to be applied to compress the solid and as a result, the density of the solid is increased, then hydrogen will transform into the lightest metal known. It will also have some fascinating properties like superconductivity at room temperature or superfluidity.
Conclusion
The thermal conductivity of hydrogen in its different states has been an interesting topic of research for many years. This article should give a comprehensive understanding of whether hydrogen is conductive or not.
Subscribe to our newsletter
& plug into
the world of circuits